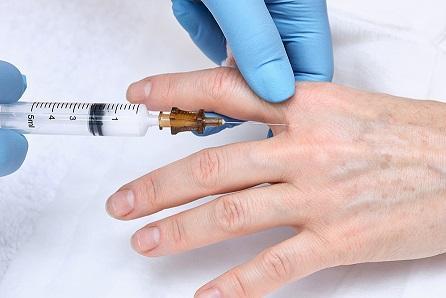
The U.S. Food and Drug Administration (FDA) has approved hyaluronic acid filler, Restylane® Lyft, for use in the hands. It is the first and only hyaluronic acid injectable gel to be FDA approved for a non-facial body part. Calcium hydroxylapatite filler, Radiesse, is approved by the FDA for use in the face and hands. According to the FDA, Restylane Lyft with lidocaine may be used in adults 22 years or older with moderate-to-severe volume deficit of the hands.
There has been an influx of dermal fillers approved by the FDA in recent years. But these aren’t just any fillers. The latest fillers target moderate to severe wrinkles and/or folds in specific anatomic locations.
Learn more about Restylane® Lyft.
Reference:
American Academy of Dermatology – www.aad.org
US Food & Drug Administration – www.FDA.gov